Chemistry 12 Unit 2 - Chemical Equilibrium Worksheet 2-2 - LeChatelier's Principle Page 2 6. Hydrogen peroxide is decomposed as follows: H2O2(l) H2(g) + O2(g) DH = +187 kJ Predict the direction of equilibrium shift by each of the following imposed changes: a) Increase the [H2].Answer _____. KMBT 654-20140224095350. LE CHATELIER'S PRINCIPLE Name Le Chatelier's Principle states that when a system at equilibrium is subjected to a stress, the system will shift its equilibrium point in order to relieve the stress. Complete the following chart by riting left, right or none r equilibrium shift, and ecreases, Increases or remains the same.

Le Chatelier's Principle Worksheet Walkthrough YouTube

le chatelier's principle Chemistry Practice Worksheet CHEM 1060 U of G Studocu

Free Printable Le Chatelier's Principle Worksheets

Equilibrium and Le Chatelier’s Principle Worksheet and Le Chatelier’s Principle Worksheet As you

Le Chatelier's Principle Practice Worksheet Answers

Le Chatelier S Principle Worksheet Answers Breadandhearth

Ap Chemistry Le Chatelier's Principle Worksheet Answers
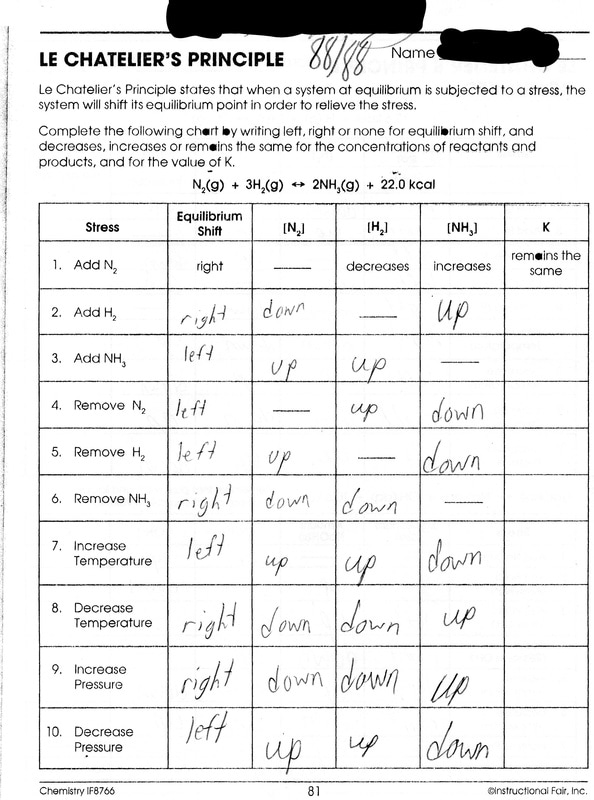
Chemistry Page 2
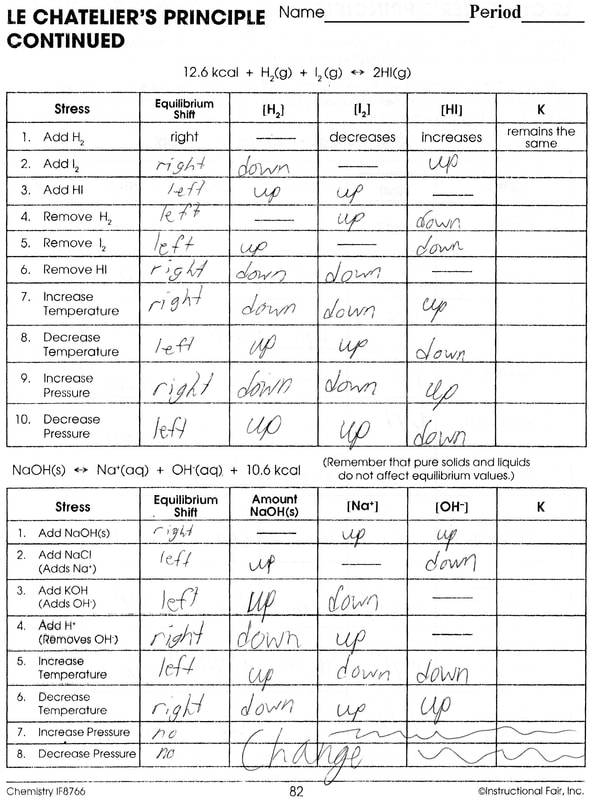
worksheet le chatelier principle answers

Le Chateliers Principle Worksheet (1) CHM 1083 Studocu

Free Le Chatelier's Principle Worksheets for Students

SOLVED Le Chatelier's Principle Worksheet For the reaction below, which change would cause the
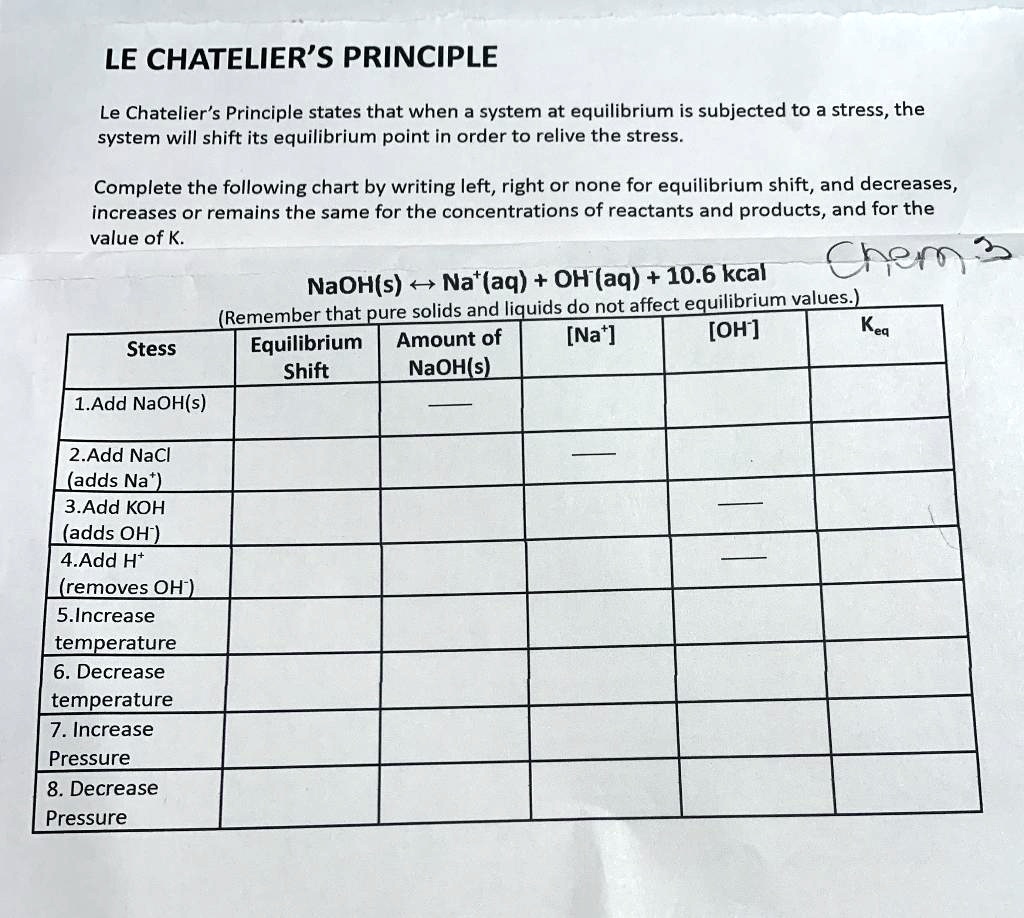
SOLVED LE CHATELIER'S PRINCIPLE Le Chatelier's Principle states that when a system at
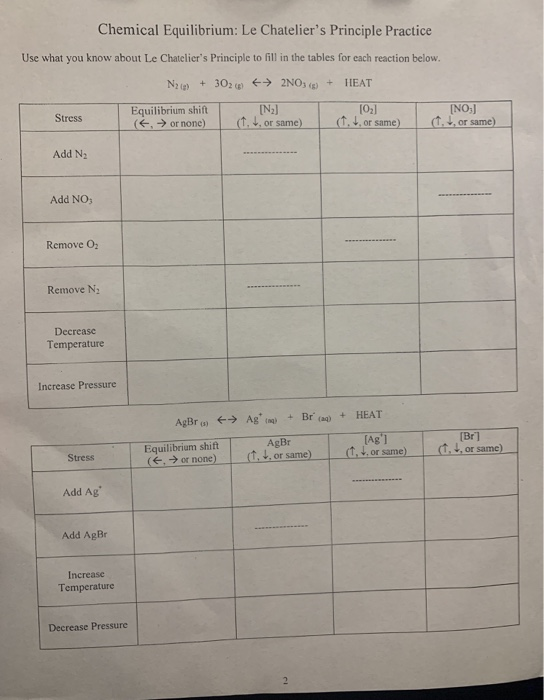
Solved Chemical Equilibrium Le Chatelier's Principle

Free Le Chatelier's Principle Worksheets for Students

Free Printable Le Chatelier's Principle Worksheets

Le Chateliers WKS KEY Dr. F Le Chatelier's Principle Worksheet KEY 1. Balance the

Solved Question 15 (1 point) Use Le Châtelier's principle to

Le Chatelier's Principle Review Worksheet Answers / Lechatlier Practice Wksht Answers Worksheet

Free Printable Le Chatelier's Principle Worksheets
Worksheet for completion to consolidate of equilibrium constants calculations. chemguide questions le principle state le principle. ethanoic acid and ethanol. Le Chatelier's Principle Worksheet KEY 1. Balance the equilibrium reaction shown below and predict whether the equilibrium will shift towards products or reactants under the changing conditions below. 2 NaHCO3 (s) + heat ↔ Na2CO3 (s) + CO2 (g) + H2O (g) the temperature is lowered: Reactants reaction is endothermic in forward direction, lower T favors reactants.